EE-Unit-I Nutrients Cycling
Inorganic nutrients occur in limited quantities and their loss to an ecosystem or retention and re-use is of great importance. The cycles of chemical elements in an ecosystem are known as nutrient cycles. If there is no loss to the ecosystem the cycle is said to be a ‘perfect cycle’ and if loss does occur the cycle is said to be ‘imperfect’. The decomposers play an important role in these cycles because they break down dead organisms and make the nutrient components available once more to other organisms.
The carbon and nitrogen cycle are two such cycles.
- The Carbon Cycle
All organic compounds contain carbon and the most important sources of all inorganic carbon is carbon dioxide in the atmosphere.
- carbon dioxide is taken up by autotrophic organisms during photosynthesis and the carbon is incorporated into carbohydrates and other compounds , such as proteins and fats;
- consumers (heterotrophic organisms) feed on plants, and their bodies assimilate carbon compounds derived from the plants;
- all organisms, including plants, release carbon dioxide during respiration as a by product. (Fermentation releases of carbon dioxide);
- when autotrophic and heterotrophic organisms die or lose body parts such as leaves, carbon dioxide is released as a result of decomposition;
- combustion of dead animal and plant material also releases carbon dioxide;
- under high pressures, dead plants and animals are carbonized, forming fossil-fuels, such as coal and crude-oil. These release carbon dioxide during combustion.
 |
|
A Diagrammatic representation of the Carbon Cycle |
- The Nitrogen cycle
Nitrogen is an element essential in all organisms, occurring in proteins and other nitrogenous compounds, e.g. nucleic acids. Although organisms live in nitrogen-rich environments (78% of the atmosphere is nitrogen) the gaseous forms of nitrogen can only be used by certain organisms. Free nitrogen must first be fixed into a useable form.
- free nitrogen in the atmosphere is mainly fixed by two groups of bacteria, nl. Azotobacter and Clostridium. The nitrogen is then used to manufacture proteins in their bodies, when they die, their proteins are broken down by decomposers (mainly bacteria and other micro-organisms), and converted into ammonia (blue-green algae, cyanobacteria, can also be use free nitrogen from the atmosphere);
- during electrical changes in the atmosphere(e.g. lightning), free nitrogen is fixed (combined) finally forming nitrate;
- nitrates are taken up by plants which use them to manufacture proteins;
- animals (herbivores) eat plants and convert plant proteins to animal proteins, while carnivores obtain their plant proteins by indirect means (by eating herbivores);
- when plants and animals die, the proteins in their bodies are broken down into ammonia by decomposers. The process is known as ammonification;
- ammonia is converted to nitrites by nitrite bacteria (Nitrosomonas and Nitrosococcus). Nitrites are again converted to nitrates by nitrate bacteria (Nitrobacter )This process is known as nitrification;
- different types of bacteria are also able to break down nitrates, nitrites and ammonia which results in the release of nitrogen. This process is known asdenitrification.
 |
|
A Diagrammatic Representation of the Nitrogen Cycle. |
Water Cycle
Earth’s water is always in movement, and the natural water cycle, also known as the hydrologic cycle, describes the continuous movement of water on, above, and below the surface of the Earth. Water is always changing states between liquid, vapor, and ice, with these processes happening in the blink of an eye and over millions of years.
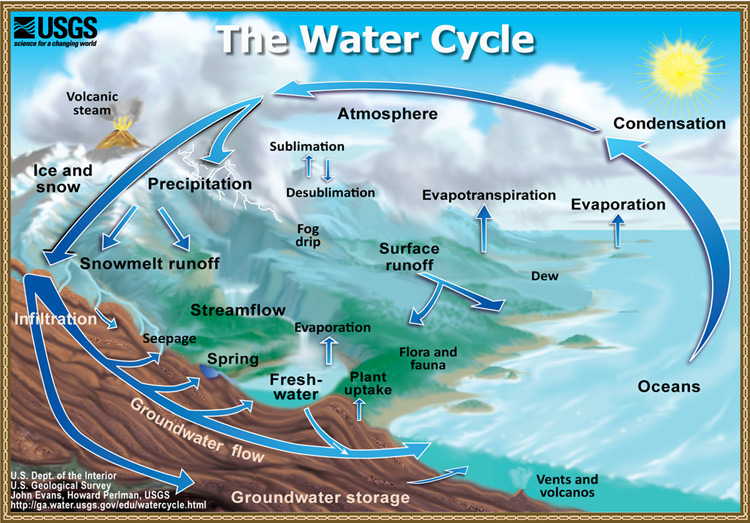
Global water distribution
For an estimated explanation of where Earth’s water exists, look at the chart below. By now, you know that the water cycle describes the movement of Earth’s water, so realize that the chart and table below represent the presence of Earth’s water at a single point in time. If you check back in a thousand or million years, no doubt these numbers will be different!
Notice how of the world’s total water supply of about 332.5 million cubic miles of water, over 96 percent is saline. And, of the total freshwater, over 68 percent is locked up in ice and glaciers. Another 30 percent of freshwater is in the ground. Fresh surface-water sources, such as rivers and lakes, only constitute about 22,300 cubic miles (93,100 cubic kilometers), which is about 1/150th of one percent of total water. Yet, rivers and lakes are the sources of most of the water people use everyday.
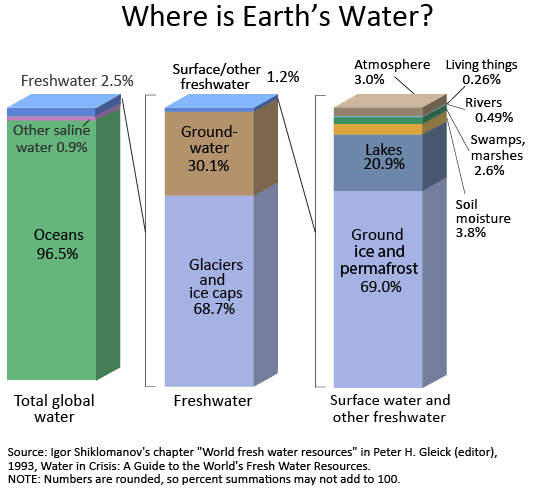
Source: Igor Shiklomanov’s chapter “World fresh water resources” in Peter H. Gleick (editor), 1993, Water in Crisis: A Guide to the World’s Fresh Water Resources (Oxford University Press, New York).
Where is Earth’s water?
For a detailed explanation of where Earth’s water is, look at the data table below. Notice how of the world’s total water supply of about 333 million cubic miles (1,386 million cubic kilometers) of water, over 96 percent is saline. And, of the total freshwater, over 68 percent is locked up in ice and glaciers. Another 30 percent of freshwater is in the ground. Thus, rivers and lakes that supply surface water for human uses only constitute about 22,300 cubic miles (93,100 cubic kilometers), which is about 0.007 percent of total water, yet rivers are the source of most of the water people use.
| Water source | Water volume, in cubic miles | Water volume, in cubic kilometers | Percent of freshwater |
Percent of total water |
|---|---|---|---|---|
| Oceans, Seas, & Bays | 321,000,000 | 1,338,000,000 | — | 96.5 |
| Ice caps, Glaciers, & Permanent Snow | 5,773,000 | 24,064,000 | 68.7 | 1.74 |
| Groundwater | 5,614,000 | 23,400,000 | — | 1.69 |
| Fresh | 2,526,000 | 10,530,000 | 30.1 | 0.76 |
| Saline | 3,088,000 | 12,870,000 | — | 0.93 |
| Soil Moisture | 3,959 | 16,500 | 0.05 | 0.001 |
| Ground Ice & Permafrost | 71,970 | 300,000 | 0.86 | 0.022 |
| Lakes | 42,320 | 176,400 | — | 0.013 |
| Fresh | 21,830 | 91,000 | 0.26 | 0.007 |
| Saline | 20,490 | 85,400 | — | 0.006 |
| Atmosphere | 3,095 | 12,900 | 0.04 | 0.001 |
| Swamp Water | 2,752 | 11,470 | 0.03 | 0.0008 |
| Rivers | 509 | 2,120 | 0.006 | 0.0002 |
| Biological Water | 269 | 1,120 | 0.003 | 0.0001 |
| Source: Igor Shiklomanov’s chapter “World fresh water resources” in Peter H. Gleick (editor), 1993, Water in Crisis: A Guide to the World’s Fresh Water Resources (Oxford University Press, New York). | ||||
Oxygen Cycle
Oxygen, like carbon and hydrogen, is a basic element of life. In addition, in the form of O3, ozone, it provides protection of life by filtering out the sun’s UV rays as they enter the stratosphere. In addition to constituting about 20% of the atmosphere, oxygen is ubiquitous. It also occurs in combination as oxides in the Earth’s crust and mantle, and as water in the oceans.
Early in the evolution of the Earth, oxygen is believed to have been released from water vapor by UV radiation and accumulated in the atmosphere as the hydrogen escaped into the earth’s gravity. Later, photosynthesis became a source of oxygen. Oxygen is also released as organic carbon in CHO, and gets buried in sediments. The role of oxygen in life is describe in the unit onBiological Systems.

Figure O1. The Oxygen Cycle
Oxygen is highly reactive. A colorless, odorless gas at ordinary temperatures, it turns to a bluish liquid at -183° C. Burning or combustion is essentially oxidation, or combination with atmospheric oxygen. Figure O1 shows a very broad overview of oxygen cycling in nature. The environment of oxygen in numerous reactions make it hard to present a complete picture.
Oxygen is vital to us in many ways (beside the most obvious–for breathing). Water can dissolve oxygen and it is this dissolved oxygen that supports aquatic life. Oxygen is also needed for the decomposition of organic waste. Wastes from living organisms are “biodegradable” because there are aerobic bacteria that convert organic waste materials into stable inorganic materials. If enough oxygen is not available for these bacteria, for example, because of enormous quantities of wastes in a body of water, they die and anaerobic bacteria that do not need oxygen take over. These bacteria change waste material into H2S and other poisonous and foul-smelling substances. For this reason, the content of biodegradable substances in waste waters is expressed by a special index called “biological oxygen demand” (BOD), representing the amount of oxygen needed by aerobic bacteria to decompose the waste.

Leave a Reply
Want to join the discussion?Feel free to contribute!