EE-Unit-III Determination of DO,BOD,COD
What is the Winkler Method?
The Winkler Method is a technique used to measure dissolved oxygen in freshwater systems. Dissolved oxygen is used as an indicator of the health of a water body, where higher dissolved oxygen concentrations are correlated with high productivity and little pollution. This test is performed on-site, as delays between sample collection and testing may result in an alteration in oxygen content.
How does the Winkler Method Work?
The Winkler Method uses titration to determine dissolved oxygen in the water sample. A sample bottle is filled completely with water (no air is left to skew the results). The dissolved oxygen in the sample is then “fixed” by adding a series of reagents that form an acid compound that is then titrated with a neutralizing compound that results in a color change. The point of color change is called the “endpoint,” which coincides with the dissolved oxygen concentration in the sample. Dissolved oxygen analysis is best done in the field, as the sample will be less altered by atmospheric equilibration.
Applications
Dissolved oxygen analysis can be used to determine:
- the health or cleanliness of a lake or stream,
- the amount and type of biomass a freshwater system can support,
- the amount of decomposition occurring in the lake or stream.
How to- Sample Collection, Preparation, Analytical Protocols, and Concerns
Dissolved oxygen should be measured as quickly and carefully as possible. Ideally, samples should be measured in the field immediately after collection.
Reagent List:
- 2ml Manganese sulfate
- 2ml alkali-iodide-azide
- 2ml concentrated sulfuric acid
- 2ml starch solution
- Sodium thiosulfate
These reagents are available in dissolved oxygen field kits, such as those made by the Hach Company. Please use caution when using these reagents, as they can be hazardous to one’s health.
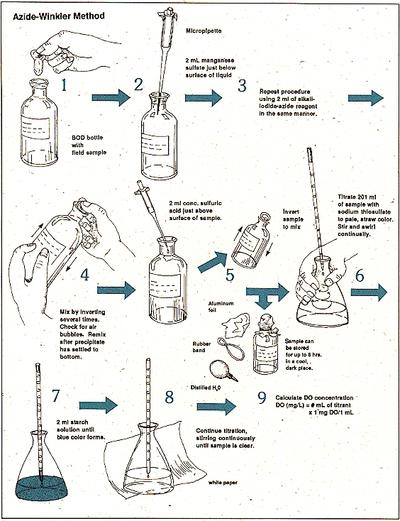
Procedure:
- Carefully fill a 300-mL glass Biological Oxygen Demand (BOD) stoppered bottle brim-full with sample water.
- Immediately add 2mL of manganese sulfate to the collection bottle by inserting the calibrated pipette just below the surface of the liquid. (If the reagent is added above the sample surface, you will introduce oxygen into the sample.) Squeeze the pipette slowly so no bubbles are introduced via the pipette.
- Add 2 mL of alkali-iodide-azide reagent in the same manner.
- Stopper the bottle with care to be sure no air is introduced. Mix the sample by inverting several times. Check for air bubbles; discard the sample and start over if any are seen. If oxygen is present, a brownish-orange cloud of precipitate or floc will appear. When this floc has settle to the bottom, mix the sample by turning it upside down several times and let it settle again.
- Add 2 mL of concentrated sulfuric acid via a pipette held just above the surface of the sample. Carefully stopper and invert several times to dissolve the floc. At this point, the sample is “fixed” and can be stored for up to 8 hours if kept in a cool, dark place. As an added precaution, squirt distilled water along the stopper, and cap the bottle with aluminum foil and a rubber band during the storage period.
- In a glass flask, titrate 201 mL of the sample with sodium thiosulfate to a pale straw color. Titrate by slowly dropping titrant solution from a calibrated pipette into the flask and continually stirring or swirling the sample water.
- Add 2 mL of starch solution so a blue color forms.
- Continue slowly titrating until the sample turns clear. As this experiment reaches the endpoint, it will take only one drop of the titrant to eliminate the blue color. Be especially careful that each drop is fully mixed into the sample before adding the next. It is sometimes helpful to hold the flask up to a white sheet of paper to check for absence of the blue color.
- The concentration of dissolved oxygen in the sample is equivalent to the number of milliliters of titrant used. Each mL of sodium thiosulfate added in steps 6 and 8 equals 1 mg/L dissolved oxygen.
Results Analysis
The total number of milliliters of titrant used in steps 6-8 equals the total dissolved oxygen in the sample in mg/L. Oxygen saturation is temperature dependent – gas is more soluble in cold waters, hence cold waters generally have higher dissolved oxygen concentrations. Dissolved oxygen also depends on salinity and elevation, or partial pressure.
The test for Biochemical Oxygen Demand is especially important in waste water treatment, food manufacturing, and filtration facilities where the concentration of oxygen is crucial to the overall process and end products. High concentrations of dissolved oxygen (DO) predict that oxygen uptake by microorganisms is low along with the required break down of nutrient sources in the medium (sample). On the other hand, low DO readings signify high oxygen demand from microorganisms, and can lead to possible sources of contamination depending on the process.
Performing the test for Biochemical Oxygen Demand requires a significant time commitment for preparation and analysis. The entire process requires five days, and it is not until the last day where data is collected and evaluated. During this time, samples are initially seeded with microorganisms and supplied with a carbon nutrient source of glucose-glutamic acid. The sample is then introduced to an environment suitable for bacterial growth at reproducible temperatures, nutrient sources, and light within a 20 degree Celsius incubator such that oxygen will be consumed. Quality controls, standards and dilutions are also run to test for accuracy and precision. Determination of the dissolved oxygen within the sample can be determined through Winkler titration methods. The difference in initial DO readings (prior to incubation) and final DO readings (after 5 days of incubation) predicts the BOD of the sample. A suitable detection limit as per environmental QC is 1 mg/L.
Chemical Oxygen Demand
In recent times, with the increase of pollution by discharging large amount of various chemicals, oxidisable organic substances of different matter enter in the aquatic system. BOD values alone does not give a clear picture of organic matter contend of the water sample. In addition, the presence of various toxicant in the sample. In addition, the presence of various toxicants in the sample may severely affect the validity of BOD test. Hence chemical oxygen demand (COD) test is a better estimate of organic matter which needs no sophistication and is time saving. However COD that is the oxygen consumed (OC) does not differentiate the stable organic matter from the unstable form, therefore the COD value are not directly comparable to that of BOD.
The amount of organic matter in water is estimated based on their oxidisability by chemical oxidants, such as potassium permanganate or potassium dichromate. For many years, the potassium permanganate was used as oxidizing agent for measuring chemical oxygen demand. But the oxidizing capacity of potassium permanganate varied widely. Nowadays, Potassium dichromate is used instead of potassium permanganate because it is more effective, relatively cheap, easy to purify and is able to oxidize almost all organic compounds.
In this method, a fixed volume of oxidant (here potassium dichromate) is added to the water sample. The organic matter present in the water sample is first oxidized with known volume of potassium dichromate and then excess of oxygen is allowed to react with potassium iodide to liberate iodine in amounts equal to the excess oxygen, which is estimated titrimetrically with sodium thiosulphate as an indicator.


Leave a Reply
Want to join the discussion?Feel free to contribute!